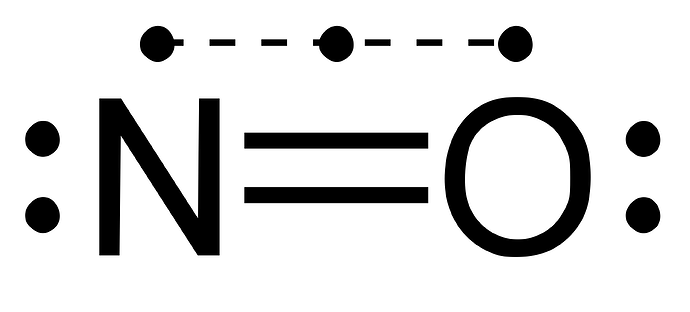![]()
![]() howdy i win!
howdy i win!
Your Lewis Structure is incorrect
It’s giving I WIN
How about this one?
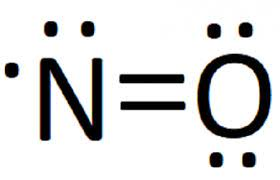
P.S. I didn’t do too well in chem this year
Closer
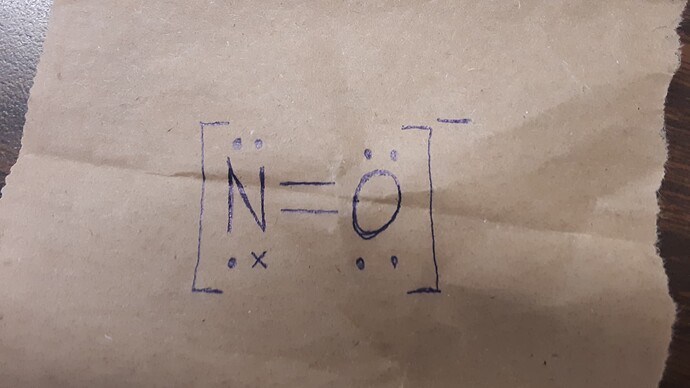
You’d add an electron to the nitrogen so that it has two lone pairs, and thus you’d make the whole polyatomic ion have a charge of minus one.
Remember, in a covalent bond all the elements are supposed to reach octet, at least in the Lewis Structures
Do you think the dot-and-cross diagram would show this more clearly?
Let me draw it for you real quick
But isn’t nitrogen monoxide/nitric oxide neutral? I’m kinda confused.
It’s not on my list of polyatomic ions, but this is the only way they both achieve octect
Hmm, true. But I heard that some molecules don’t obey the octet rule and this may be one of them because NO has an odd number of electrons.
That’s why ions exist
As far as I’m aware, beither nitrogen nor oxygen are exceptions
ions have existed for eons
bad dad joke for you all as i take the win
Only eons?
I win
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
(Don’t mind the shoes, it just built different)
Good job @Legend ! ![]()
⠛⠛⣿⣿⣿⣿⣿⡷⢶⣦⣶⣶⣤⣤⣤⣀⠀⠀⠀
⠀⠀⠀⣿⣿⣿⣿⣿⣿⣿⣿⣿⣿⣿⣿⣿⣿⣷⡀⠀
⠀⠀⠀⠉⠉⠉⠙⠻⣿⣿⠿⠿⠛⠛⠛⠻⣿⣿⣇⠀
⠀⠀⢤⣀⣀⣀⠀⠀⢸⣷⡄⠀⣁⣀⣤⣴⣿⣿⣿⣆
⠀⠀⠀⠀⠹⠏⠀⠀⠀⣿⣧⠀⠹⣿⣿⣿⣿⣿⡿⣿
⠀⠀⠀⠀⠀⠀⠀⠀⠀⠛⠿⠇⢀⣼⣿⣿⠛⢯⡿⡟
⠀⠀⠀⠀⠀⠀⠀⠀⠀⠀⠦⠴⢿⢿⣿⡿⠷⠀⣿⠀
⠀⠀⠀⠀⠀⠀⠀⠙⣷⣶⣶⣤⣤⣤⣤⣤⣶⣦⠃⠀
⠀⠀⠀⠀⠀⠀⠀⢐⣿⣾⣿⣿⣿⣿⣿⣿⣿⣿⠀⠀
⠀⠀⠀⠀⠀⠀⠀⠈⣿⣿⣿⣿⣿⣿⣿⣿⣿⡇⠀⠀
⠀⠀⠀⠀⠀⠀⠀⠀⠀⠙⠻⢿⣿⣿⣿⣿⠟⠁